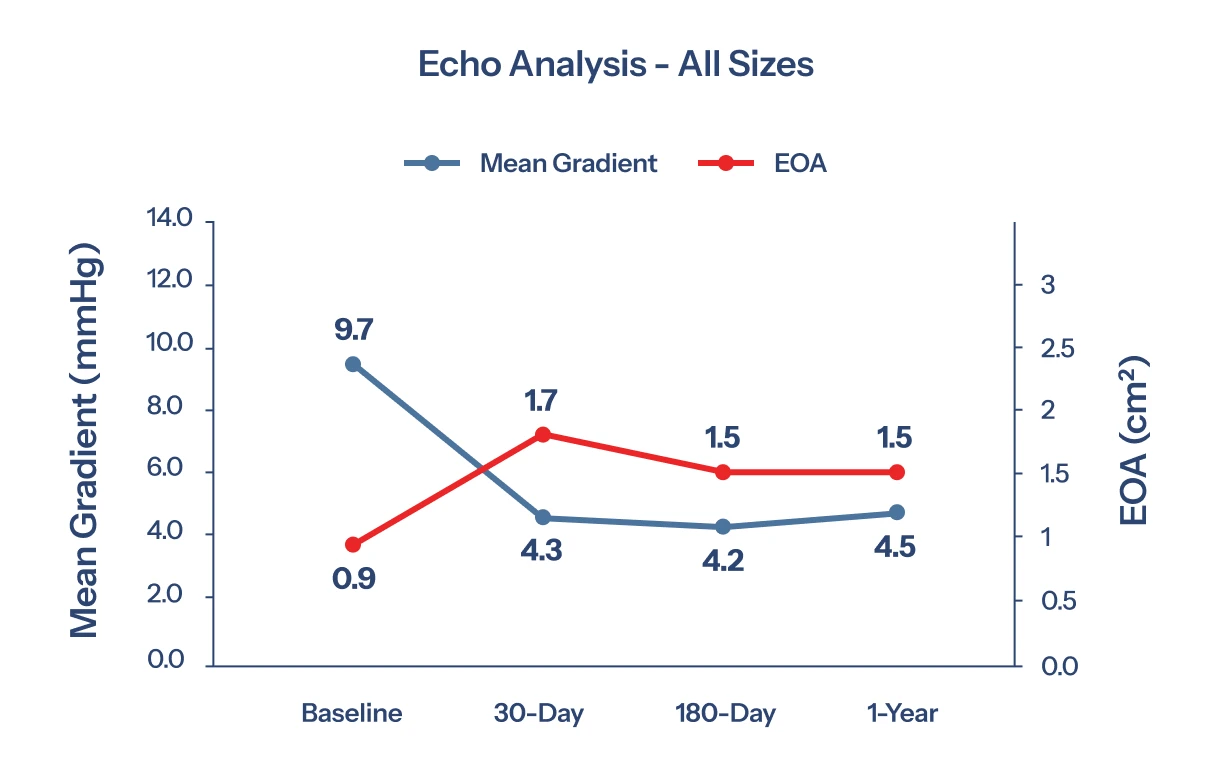
Compelling 1 year results demonstrate a good safety profile, sustained hemodynamic performance, and statistically significant improvement in patient quality-of-life. 5



(India Clinical Trail)5 N=67
N=30
All-Cause Mortality
6 (9.1%)
4 (13.3%)
Valve Reintervention
0 (0%)
0 (0%)
Structural Valve Deterioration
0 (0%)
0 (0%)
(India Clinical Trail) N=67
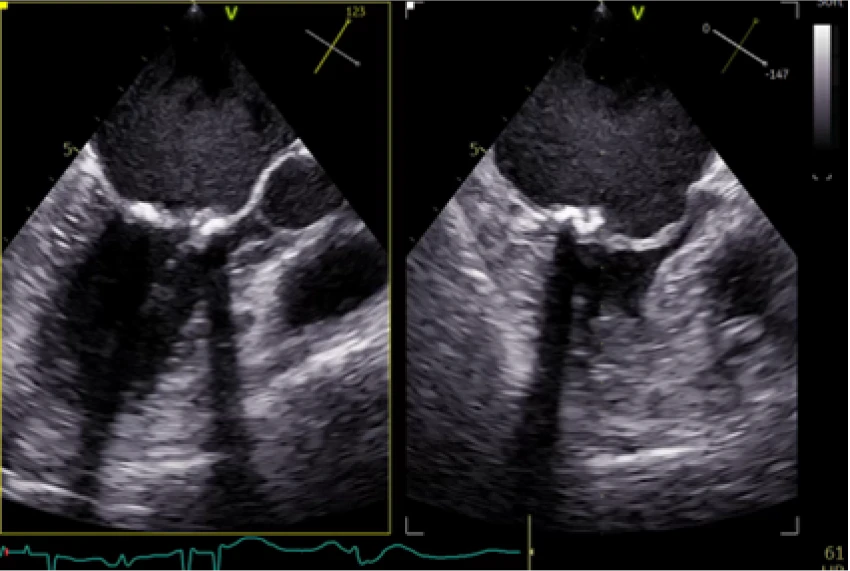

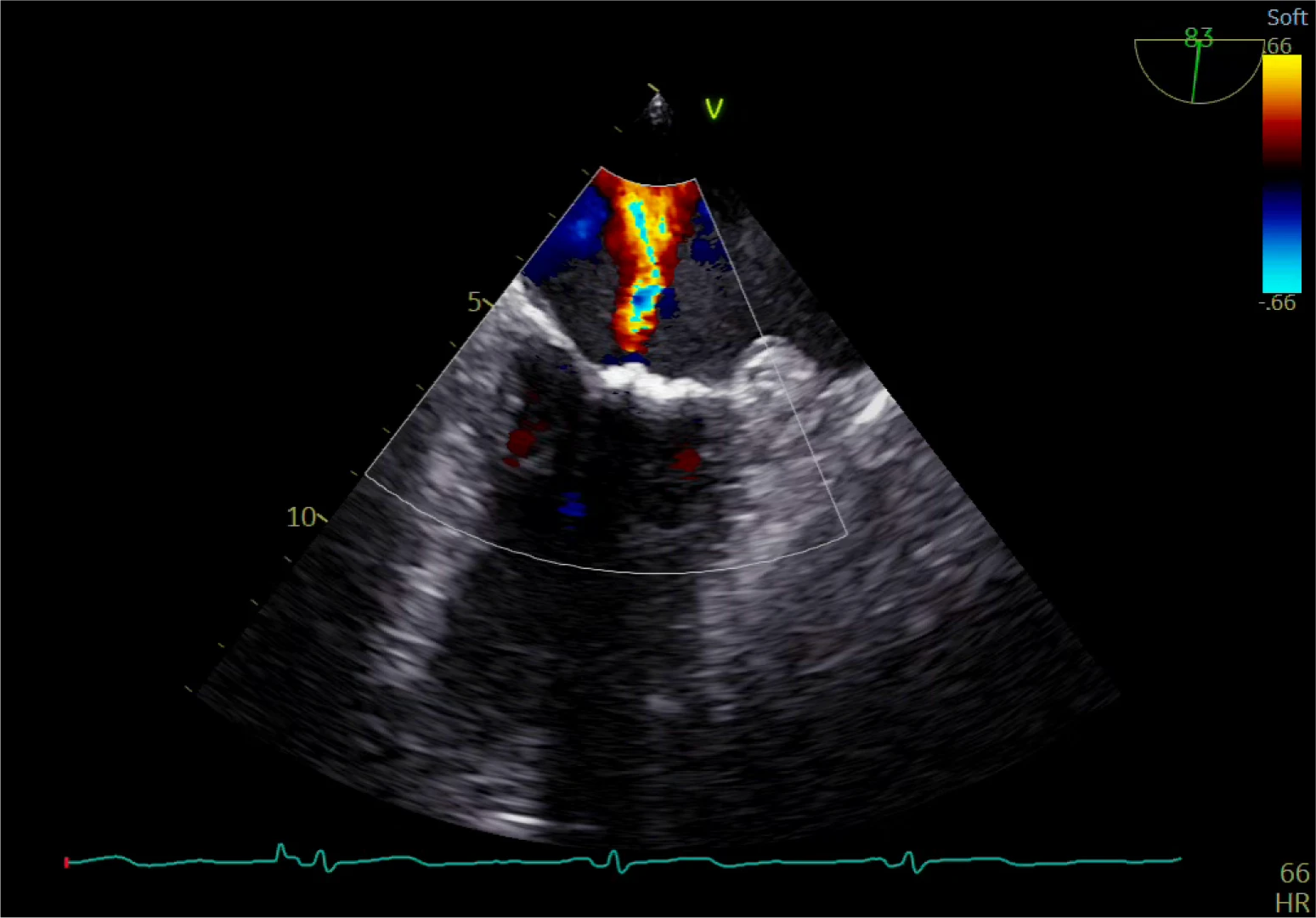
Pre-Implant
Restricted flow through valve
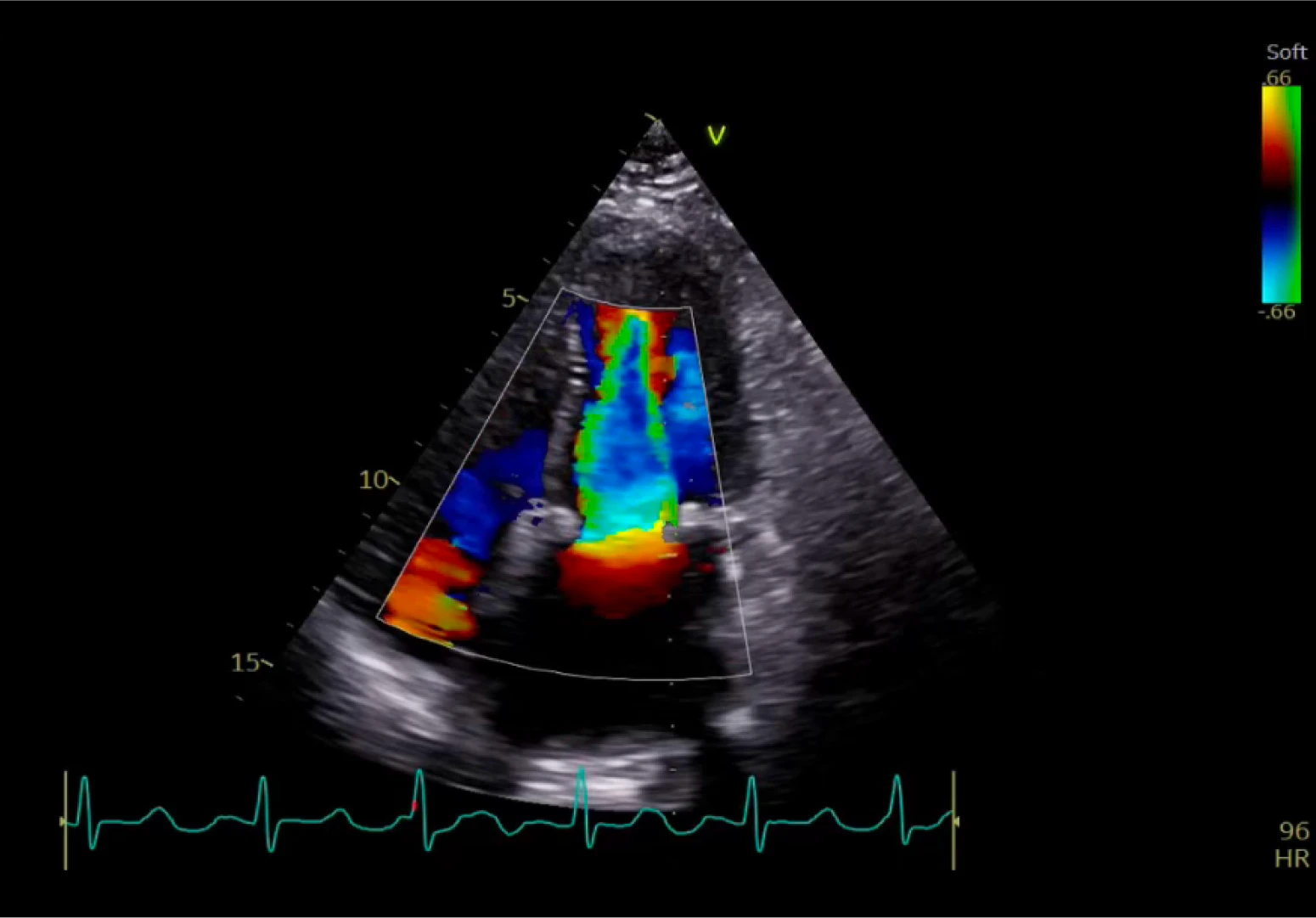
Post-Implant
Good flow through valve
“Before the TRIA™ Mitral Valve, patients faced tough choices: tissue valves made from porcine or pericardial tissue with limited durability and cultural concerns in India, or mechanical valves that audibly click and require lifelong blood thinners. With increasing positive clinical outcomes, the TRIA™ Mitral Valve is emerging as a transformative option for patients of all ages with mitral valve disease of any etiology.”

"Offering the TRIA™ Mitral Valve to women of child-bearing age is incredibly rewarding. This valve is designed to provide a lifetime solution without the possible need for long-term blood thinners or it may not require lifetime anticoagulation. With the TRIA™ Mitral Valve, women can embrace the hope of having children while avoiding the challenges of repeat surgeries."

“The TRIA™ Mitral Valve provides excellent and sustained hemodynamic performance, maintaining stable gradients and efficient blood flow over time. This consistency is critical for optimizing patient outcomes and long-term valve function.”
